2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1.90 and 200 atm is 1.10.A certain mass of Noccupies a volume of 1
5 (216) In stock

Click here:point_up_2:to get an answer to your question :writing_hand:2 112153 1215 jals 42 5the compressibility factor for nitrogen at 330 k and 800
Click here👆to get an answer to your question ✍️ -2- 1-12-15 -3- 12-15- Jals -4- 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1-90 and 200 atm is 1-10-A certain mass of Noccupies a volume of 1 dmat 330 Kand eoo atm calculate volume occupied by same cuany of gas 750 K and 200 atm- -1- 1 L -2- 2L -3- 3L

Solved 2.86 Find the compressibility factor for nitrogen at

The compressibility factor of nitrogen at 400 K and 800 atm is
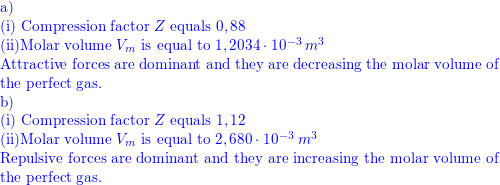
a) A gas at 250 K and 15 atm has a molar volume 12 per cent
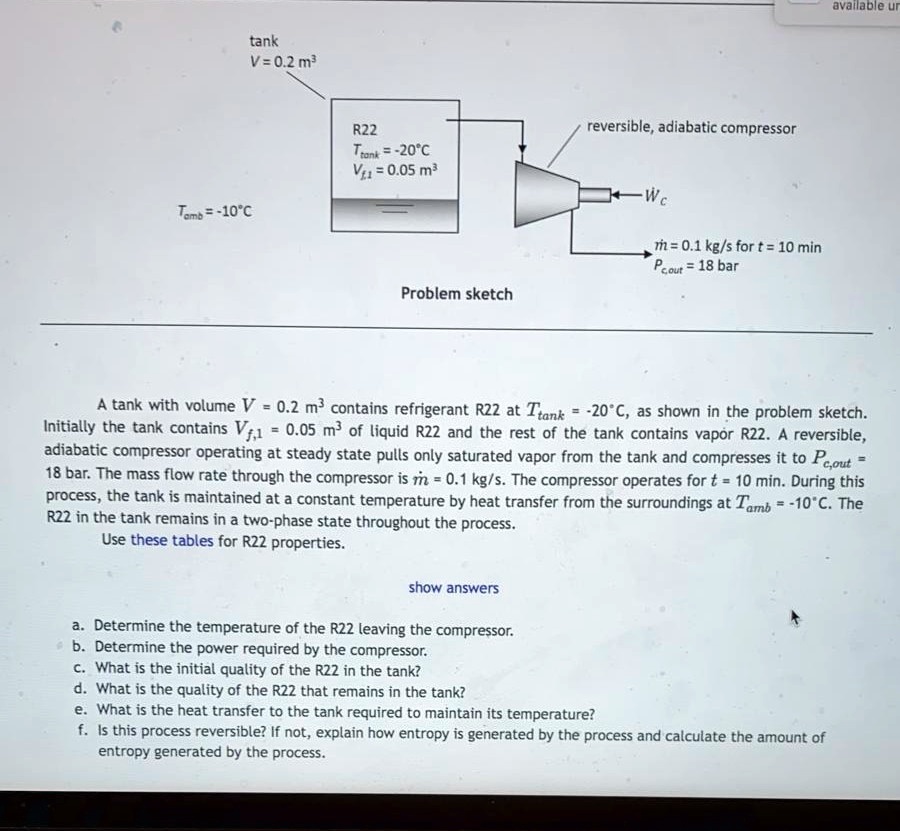
SOLVED: Text: available u tank V=0.2 m^3 R22 Tan=-20°C V=0.05 m^3
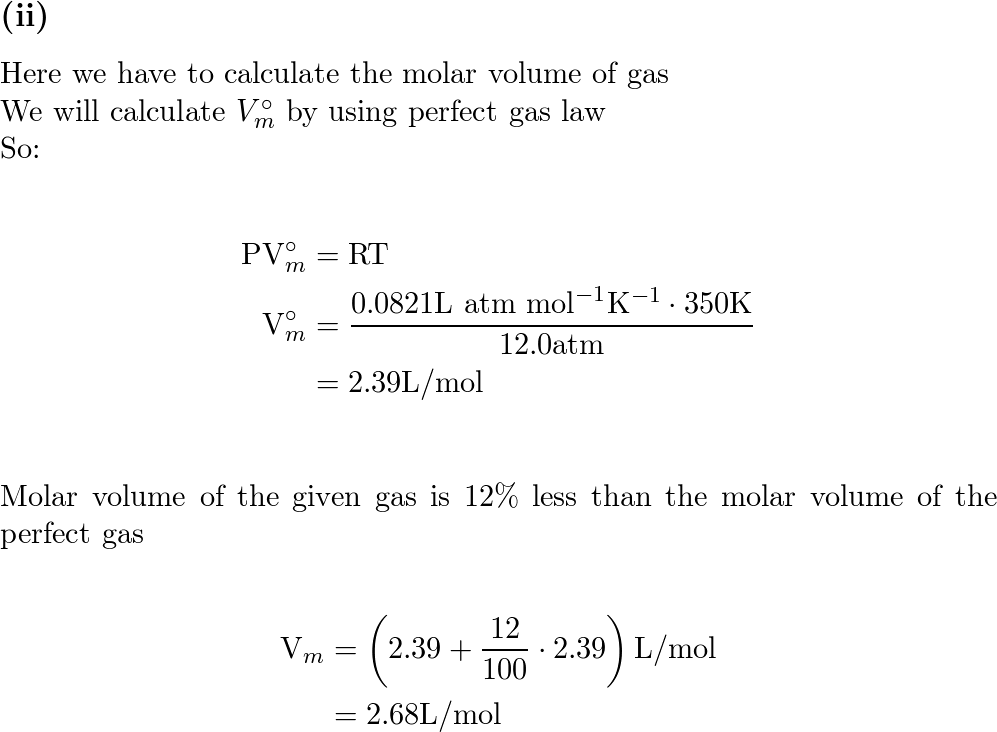
a) A gas at 250 K and 15 atm has a molar volume 12 per cent
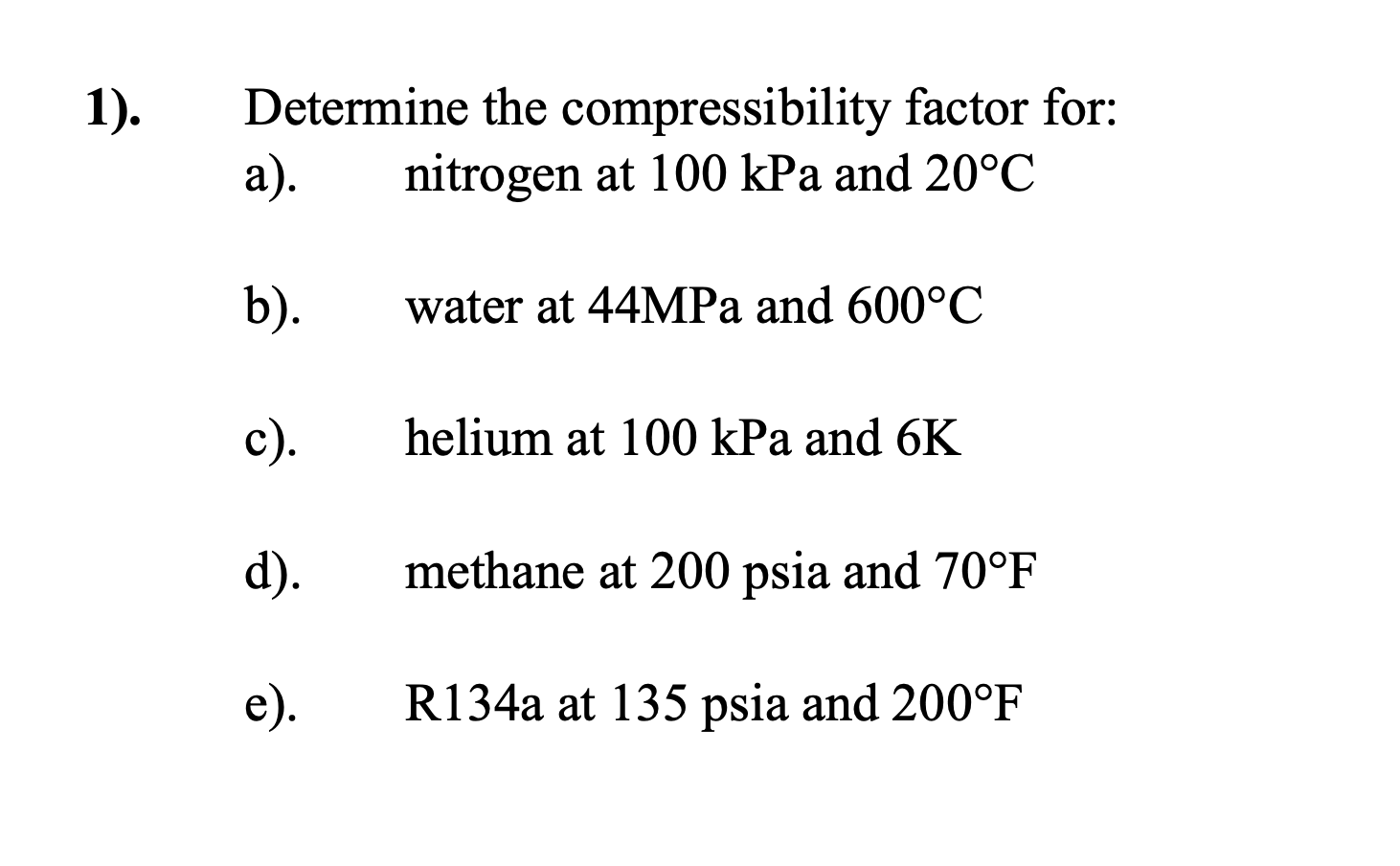
Solved Determine the compressibility factor for: a).
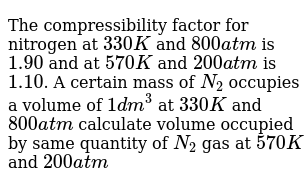
The compressibility factor for nitrogen at 330K and 800 atm is 1.90 an
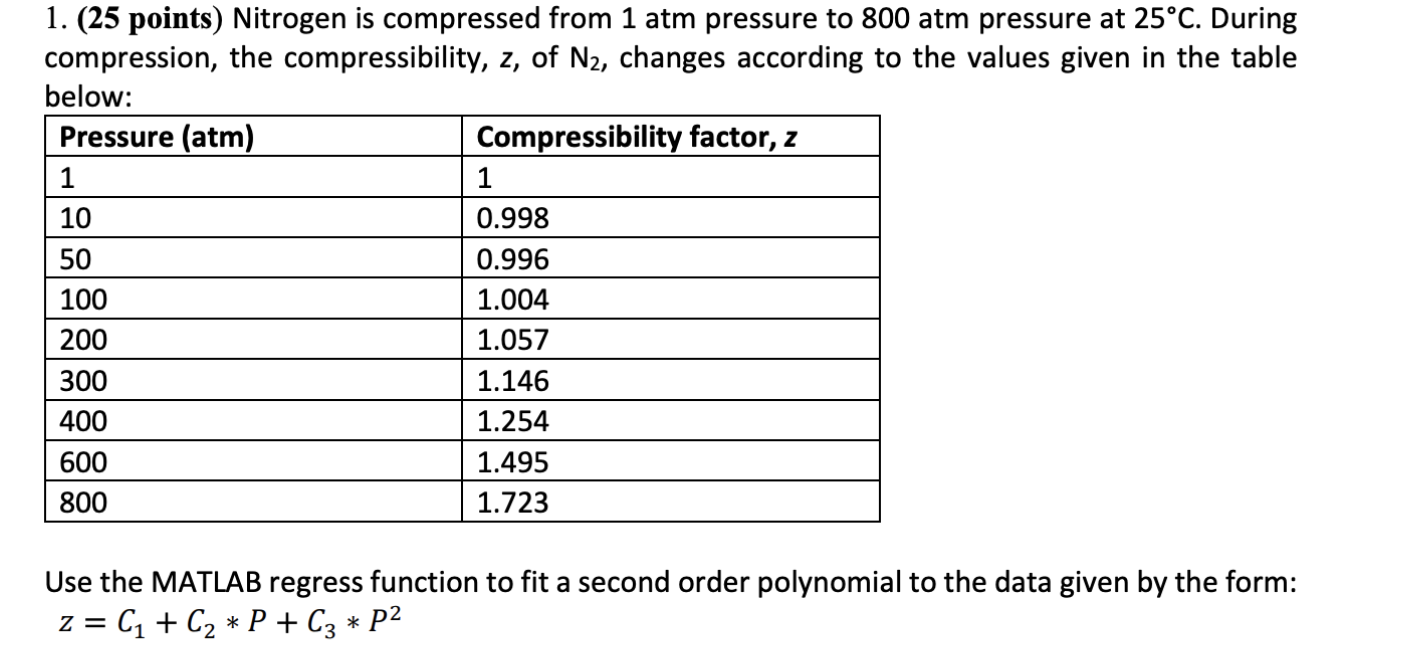
Solved 1. (25 points) Nitrogen is compressed from 1 atm

The compressibility factor for nitrogen at 330 K and 800 atm is
What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
Answered: The virial equation of state gives the…
Physical Chemistry The Compression Factor (Z) [w/1 example
3.2 Real gas and compressibility factor – Introduction to
Thermo] Derivation of compressibility factor vs reduced pressure
 Flash Icon Symbol Simple Design Royalty Free SVG, Cliparts
Flash Icon Symbol Simple Design Royalty Free SVG, Cliparts Roxy Womens Kaileo High Waisted Ankle Length Workout Leggings Size
Roxy Womens Kaileo High Waisted Ankle Length Workout Leggings Size Ready Steady Medium Impact Wire-Free Sports Bra
Ready Steady Medium Impact Wire-Free Sports Bra Esta es la versión de pantalones de lino que se convertirá en tu
Esta es la versión de pantalones de lino que se convertirá en tu Offering High-Quality Silicone Couplers - 2.000 ID 90 Degree, 10
Offering High-Quality Silicone Couplers - 2.000 ID 90 Degree, 10 Buy Crop Sweatshirt by Inks on
Buy Crop Sweatshirt by Inks on