For H(2) gas, the compressibility factor,Z = PV //n RT is
4.5 (179) In stock

For H(2) gas, the compressibility factor,Z = PV //n RT is

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Compressibility factor, Z of a gas is given as `Z=(pV)/(nRT)` (i) What is the value of Z for an

34. What is Compressibility factor? [Imp.Q] A: The ratio of the actual m..

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Non-Ideal Gas Behavior Chemistry: Atoms First
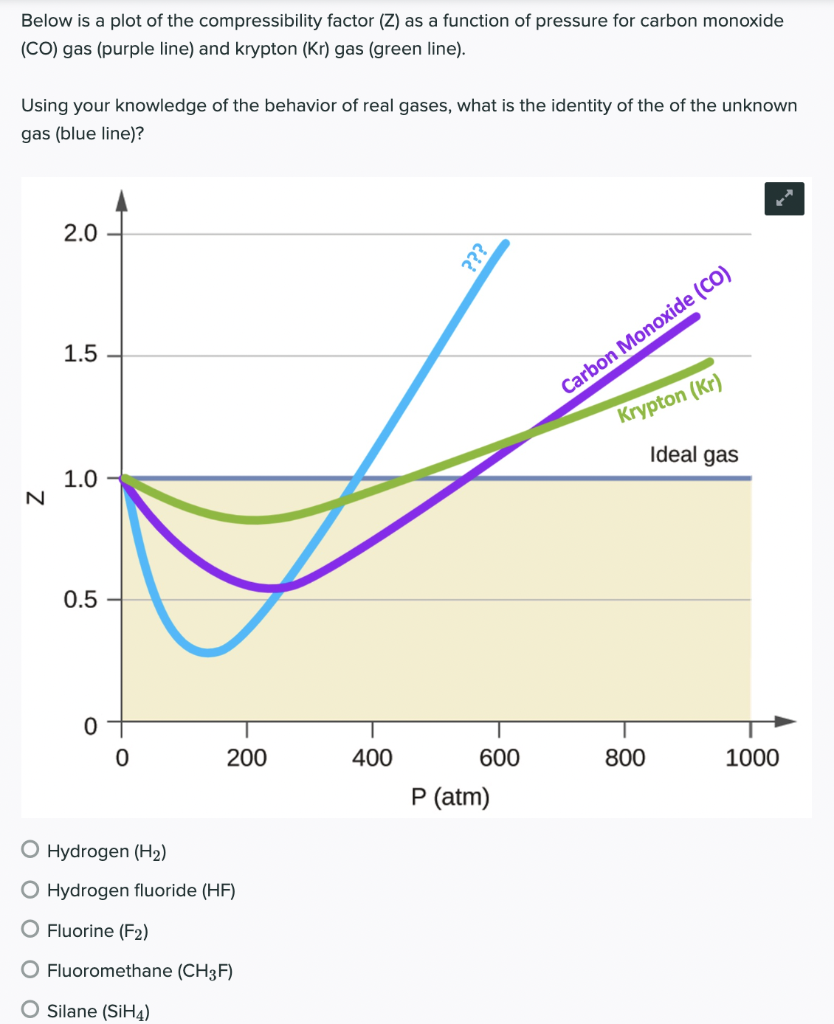
Solved Below is a plot of the compressibility factor (Z) as

SOLUTION: State of matter gases liquids and solids - Studypool

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0-b) = RT may be written as (P+*}() =RT of PV + 9 =RT of PV=RT - For large V (at very
Why there is different between the value of compressibility factor at critical point between real and ideal gas? - Quora

SOLVED: The ideal gas equation of state can be written using the compressibility factor as Z=(P V)/(R T)=1 Many improvements of this equation focus on adding dimensionless volumetric or density correction terms

e Compressibility factor (Z) for hydrogen WRT pressure and temperature
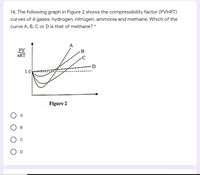
Answered: 14, The following graph in Figure 2…
Excel Calculations: Compressibility Factor for Natural Gas
At a high pressure, the compressibility factor (Z) of a real gas is us
Gas Compressibility Factor Spreadsheet Calculator
Thermodynamics - 3-7 Ideal Gas Equation with compressibility
The compression factor (compressibility factor) one mole of a van
 Buy NIKE Pro Classic Padded Womens Sports Bra (Small, Barely Volt/Seaweed/ White) Online at desertcartZimbabwe
Buy NIKE Pro Classic Padded Womens Sports Bra (Small, Barely Volt/Seaweed/ White) Online at desertcartZimbabwe Body Shaper Girdle Calzones High Compression Bbl Post Op Surgery
Body Shaper Girdle Calzones High Compression Bbl Post Op Surgery Casey Kevin Jockstraps for Men Jock Strap Wide Waistband Athletic Supporters Sexy Underwear : : Clothing, Shoes & Accessories
Casey Kevin Jockstraps for Men Jock Strap Wide Waistband Athletic Supporters Sexy Underwear : : Clothing, Shoes & Accessories Women's Elila 2101 Stretch Lace Overlay Microfiber Underwire Bra (Black 44I)
Women's Elila 2101 Stretch Lace Overlay Microfiber Underwire Bra (Black 44I) 4 Layer Menstrual Panties for Women Period Panties Absorbent Panties for Period Leakproof Period Underwear Culotte Menstruelle
4 Layer Menstrual Panties for Women Period Panties Absorbent Panties for Period Leakproof Period Underwear Culotte Menstruelle Ladies Chase Elliott Sweatshirts, Chase Elliott NASCAR Hoodies
Ladies Chase Elliott Sweatshirts, Chase Elliott NASCAR Hoodies