Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations
4.8 (110) In stock

This draft guidance document reflects Health Canada’s current thinking on Investigational Testing Authorizations (ITA) for medical devices and may be subject to changes as policy develops. The document clarifies application requirements and processes, including pre-ITA meetings, format for an ITA application and filing requests for revisions to an ITA.

Canada's Health Canada - Global Regulatory Partners, Inc.

RQM+ Medical Device and In Vitro Diagnostic Blog

Canada's Health Canada - Global Regulatory Partners, Inc.

Guidance Document - Creation of the Canadian Module 1 Backbone
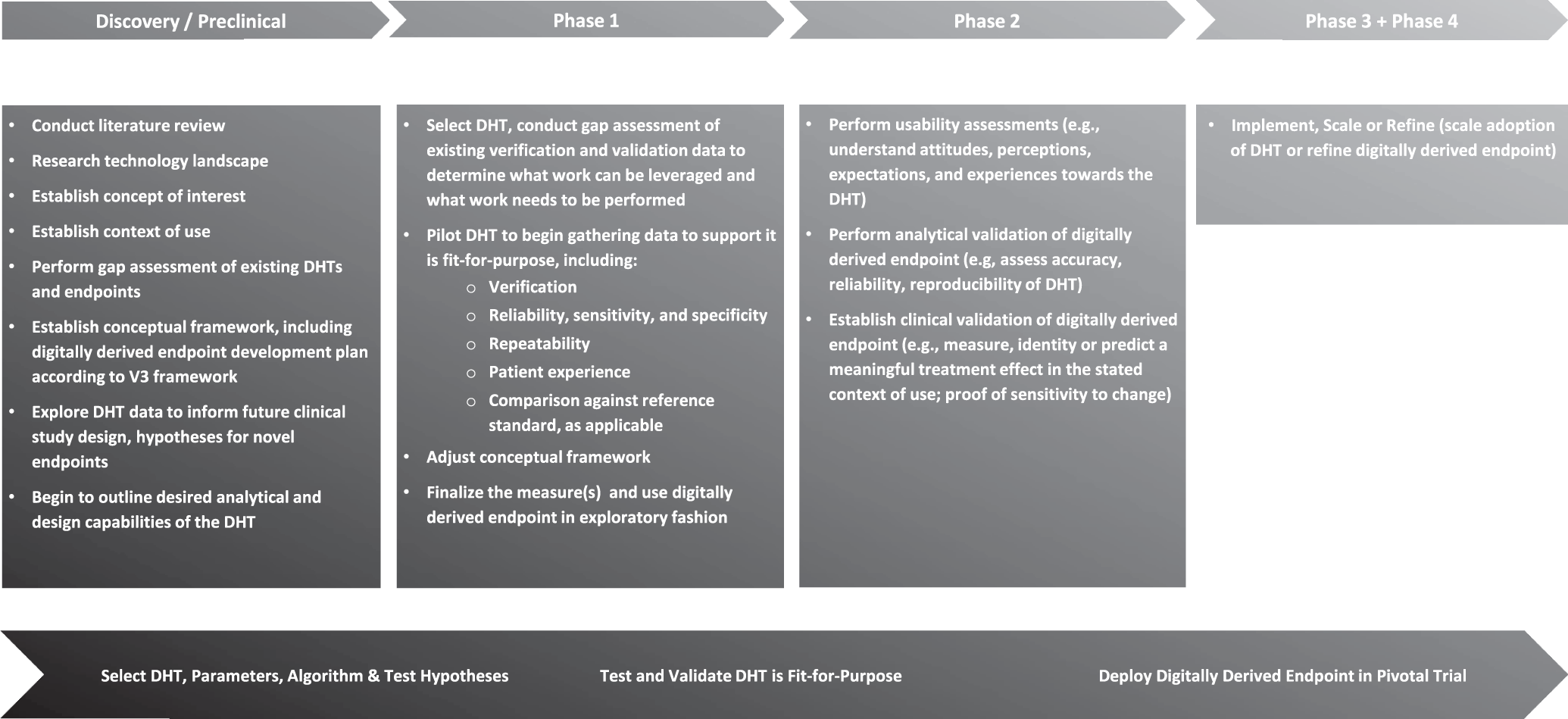
Incorporating digitally derived endpoints within clinical development programs by leveraging prior work
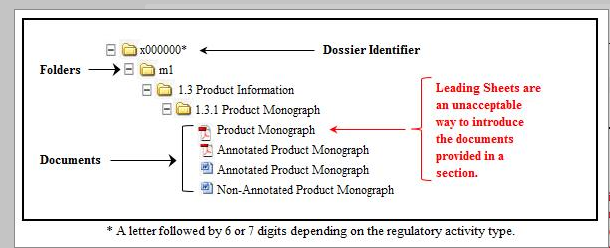
Guidance document: preparation of regulatory activities in non-eCTD format
%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png?width=4250&name=(cover)%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png)
The Difference Between Intended Use and Indications of Use (And Why These Statements Are So Important)
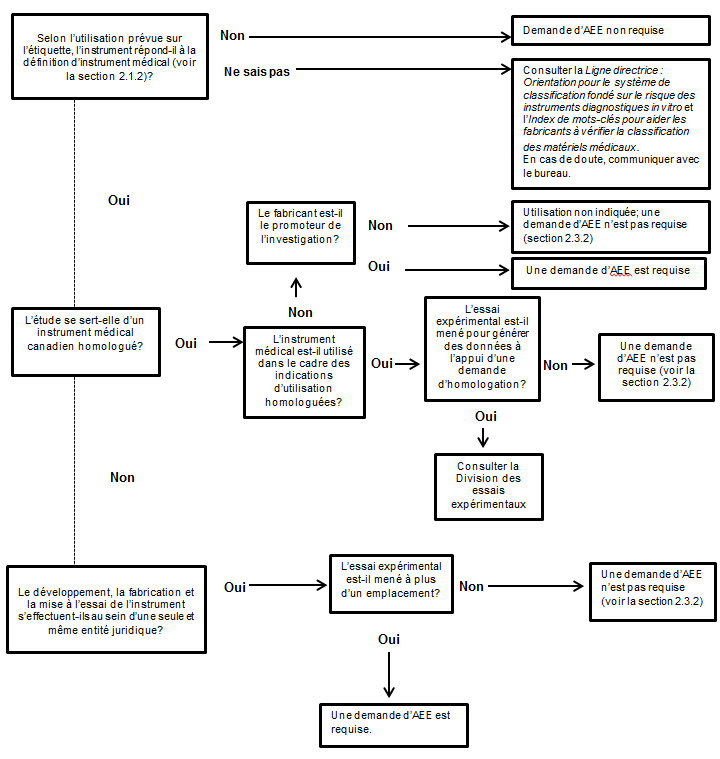
Ebauche de la Ligne directrice : Demandes d'autorisation d'essai

Applications for Medical Device Investigational Testing Authorizations Guidance Document
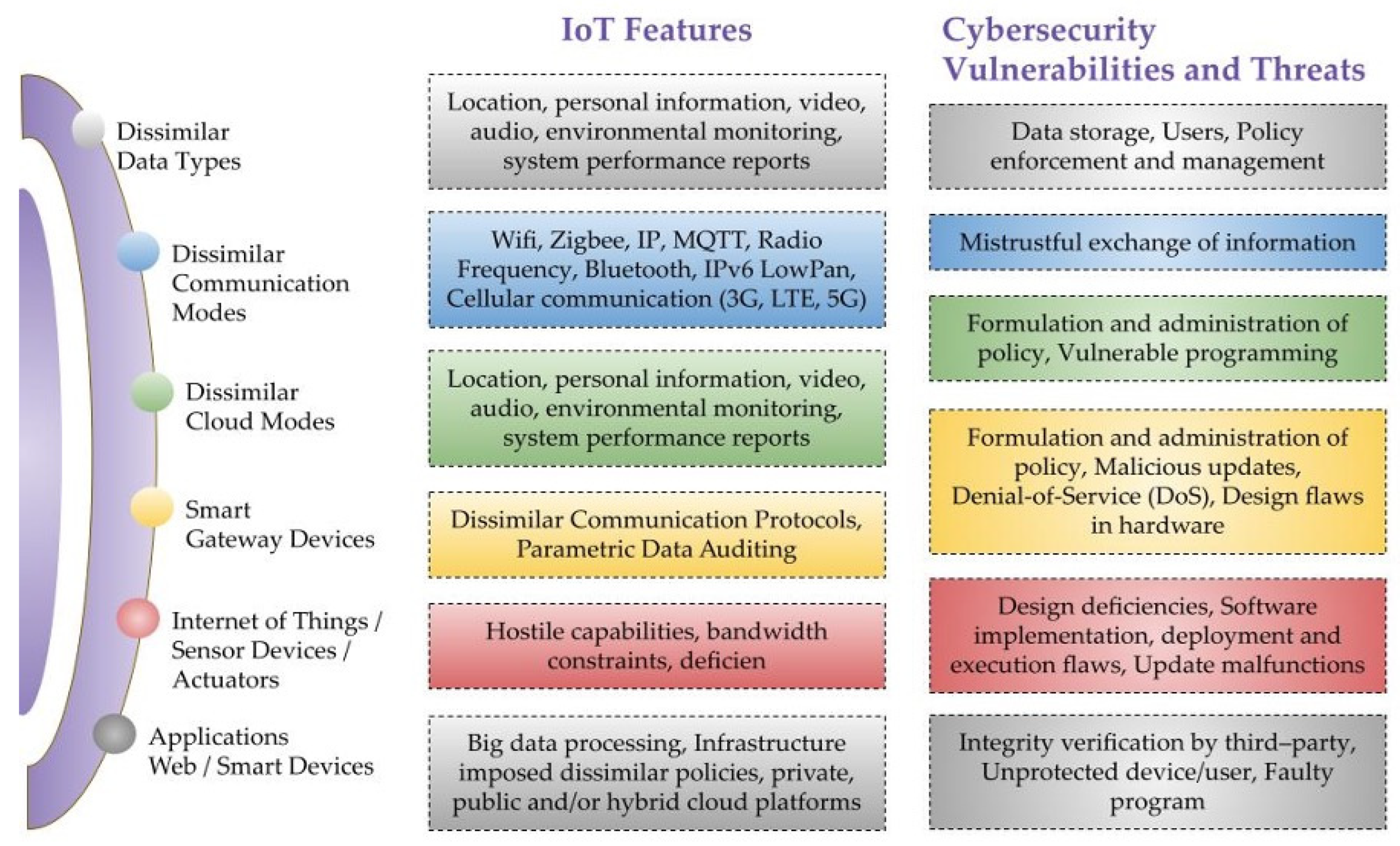
Sensors, Free Full-Text

The evolution of Canada's medical device regulatory framework

Canada's Health Canada - Global Regulatory Partners, Inc.
ITA-MED Fingertip Deluxe Pulse Oximeter with 6-way OLED display
ITA-MED Breathable Duo-Adjustable Back Support with Back Pocket
 Sports Clothing Catalog Template in InDesign, Word - Download
Sports Clothing Catalog Template in InDesign, Word - Download Flarixa Waist Trainer Body Shaper Women High Waist Flat Belly Shaping Panties Plus Size Tummy Control Shorts Slimming Underwear
Flarixa Waist Trainer Body Shaper Women High Waist Flat Belly Shaping Panties Plus Size Tummy Control Shorts Slimming Underwear Zella Live In Jogger Pants - Black - Large Womens Yoga Athleisure
Zella Live In Jogger Pants - Black - Large Womens Yoga Athleisure Women's Essential Training Sports Bra, Blue
Women's Essential Training Sports Bra, Blue Women 4 Piece Rash Guard Long Sleeve Zipper Bathing Suit Tops
Women 4 Piece Rash Guard Long Sleeve Zipper Bathing Suit Tops Gloria Vanderbilt Women's Plus Size Amanda Classic High Rise Tapered Jean
Gloria Vanderbilt Women's Plus Size Amanda Classic High Rise Tapered Jean