How to Calculate Normality of a Solution
4.9 (660) In stock
:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)
The normality of a solution is the gram equivalent weight of a solute per liter of solution. Here are examples of how to calculate the normality.

How to Calculate Normality: 4 Steps (with Pictures) - wikiHow

Molarity, Molality, Normality, Part per million (ppm) and other basic terms of Concentration solution with definition & formula, Chemistry Basic, 02, by Amrita Shetty

Normality - Formula, Definition, Calculations [Solved Examples] - Edureify-Blog

Standardization of Acid Solution: Calculate the normality of a solution of `H_(2)SO_(4)` if 40.0 mL

define the normality 5 gram of substance is dissolved in 1000 ml of caustic soda solution calculate the
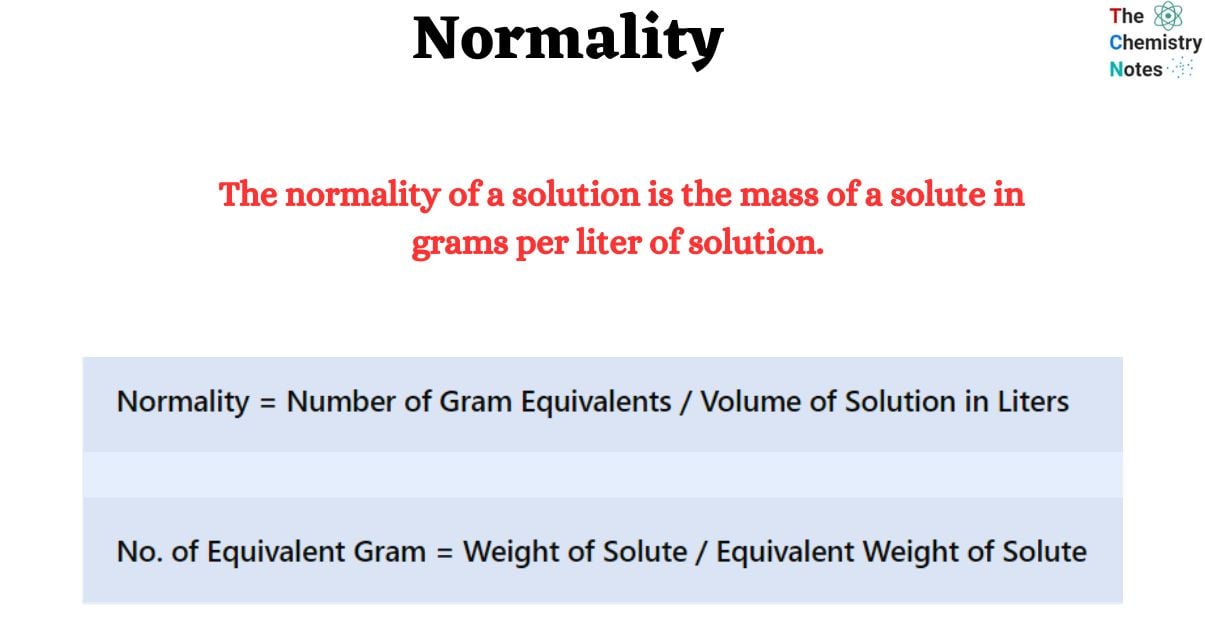
Normality: Formula, Uses, Calculation, Limitation

Normality Calculation - Chemistry

My Smart Class : How To Calculate Normality

The normality of solution containing 31.5g of hydrated oxalic acid (H₂C₂O₄.2H₂O) - NEETLab
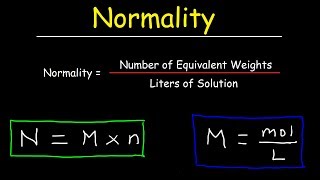
How To Calculate Normality & Equivalent Weight For Acid Base Reactions In Chemistry
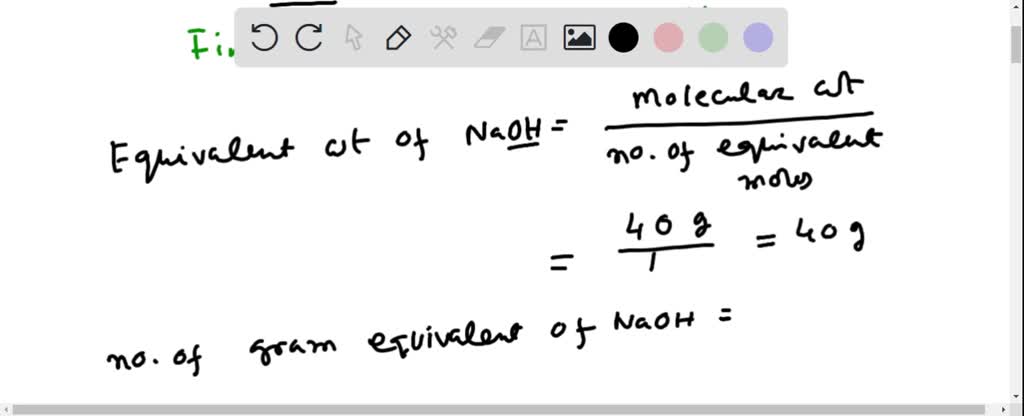
SOLVED: Calculate the normality of NaOH solution formed by dissolving 0.2 gm NaOH to make 250 ml solution: Select one: 2.0N 2.5N 0.2N 18N
The number of ions present in 0.2 mole of sodium chloride is
Health Assessment Handout 2, PDF, Blood Pressure
BARCHART NURSING TERMINOLOGY Brazosport College Campus Store
SOLVED: 3. “Normal” body temperature varies by time of day. A
Stupell Industries Conversion Chart Metrics to Imperial Unit By
 Pinsy Shapewear High Neck Hourglass Shapewear Cheeky Bodysuit
Pinsy Shapewear High Neck Hourglass Shapewear Cheeky Bodysuit Gym Shark Leggings Womens Medium Green Stretch Seamless Training Gym Yoga
Gym Shark Leggings Womens Medium Green Stretch Seamless Training Gym Yoga Eave Notching - Standing Seam Panels
Eave Notching - Standing Seam Panels- Velform Cross Compression Shaper 2x1 - Body Shaper
- ASOS DESIGN hair clip claw in black
 Cuoff Y-2k Crop Top U-rban Seamless Top Outfitters Dupes Y-2k Baby Tees for Women Baby Crop Tees -Crew Neck Go for Gold Seamless Top
Cuoff Y-2k Crop Top U-rban Seamless Top Outfitters Dupes Y-2k Baby Tees for Women Baby Crop Tees -Crew Neck Go for Gold Seamless Top
