My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com
4.8 (228) In stock

• Compressibility factor z: ▪ Dimensionless quantity. ▪ For a pure substance, it is a function of temperature and pressure or temperature and molar volume. ▪

Abhijit Roy - Performing Democracy in Culture and Politics in South Asia - Performative Communication-Routledge (2018)

General Chemistry 1B. Lecture 4. Intermolecular Forces Liquids & Solids, Part IV
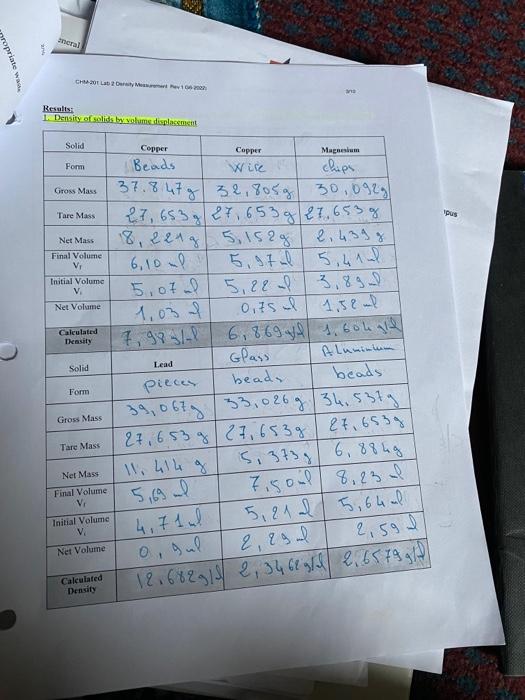
Solved CHM-201 General Chemistry and Laboratery I Laboratory
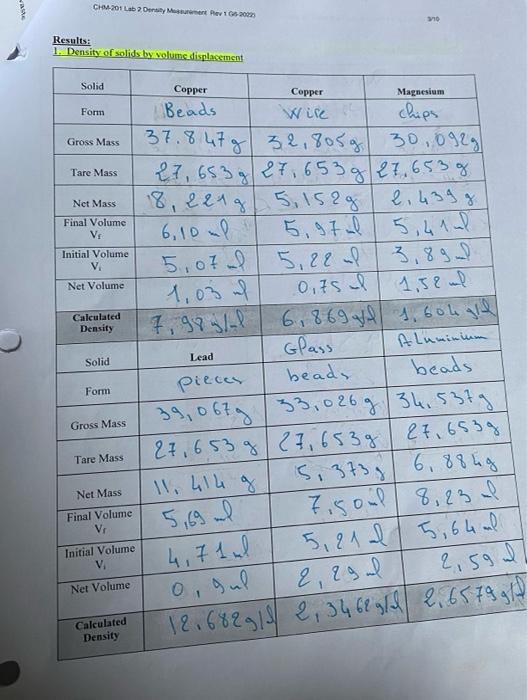
Solved CHM-201 General Chemistry and Laboratery I Laboratory

My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created

CM1000, CM1002, CM2101 First file of lecture overheads for Organic

Unit 1 (formerly Module 2) - ppt download
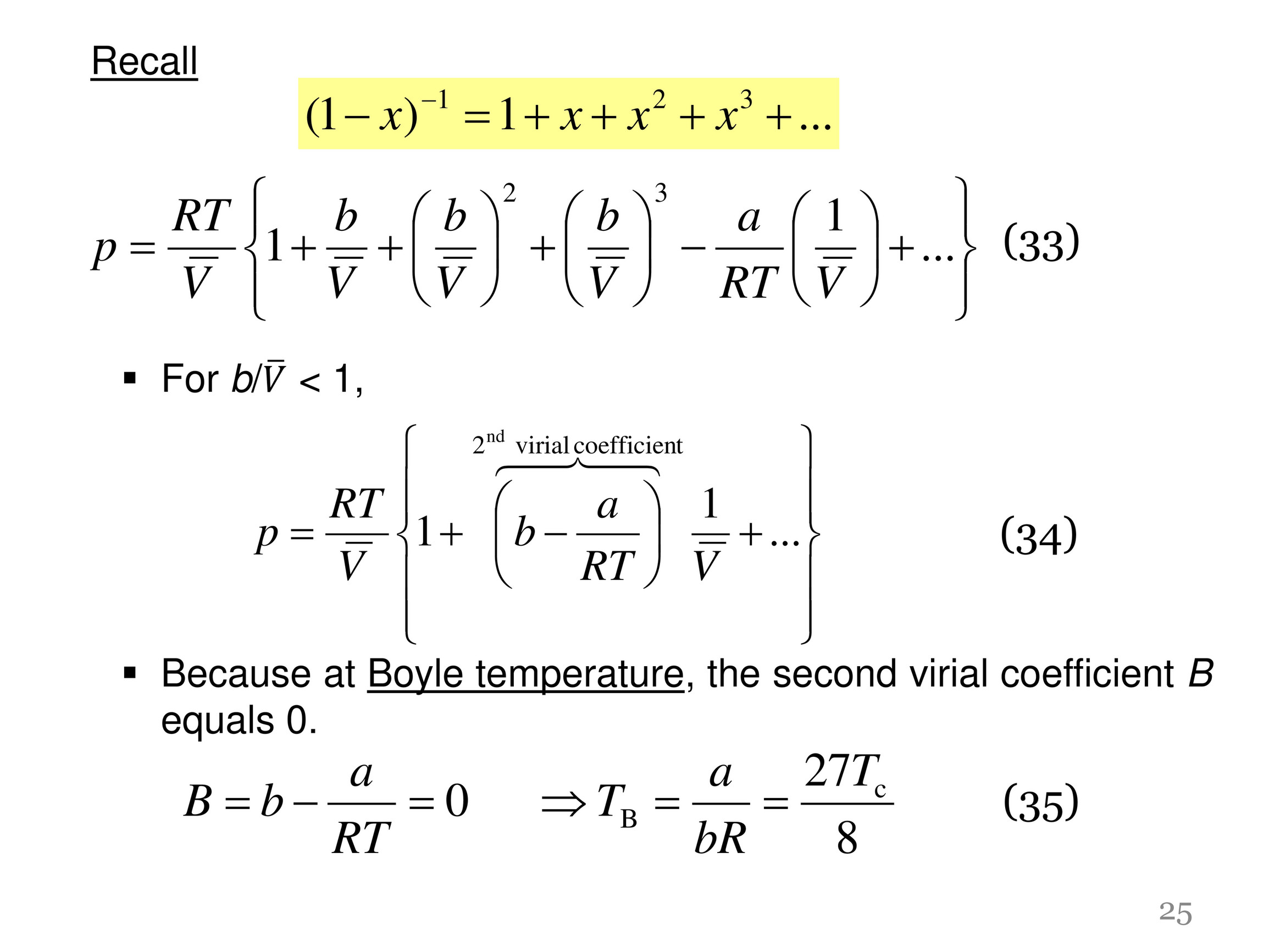
My publications - CHM 201-LECTURE IV-REAL GASES - Page 25
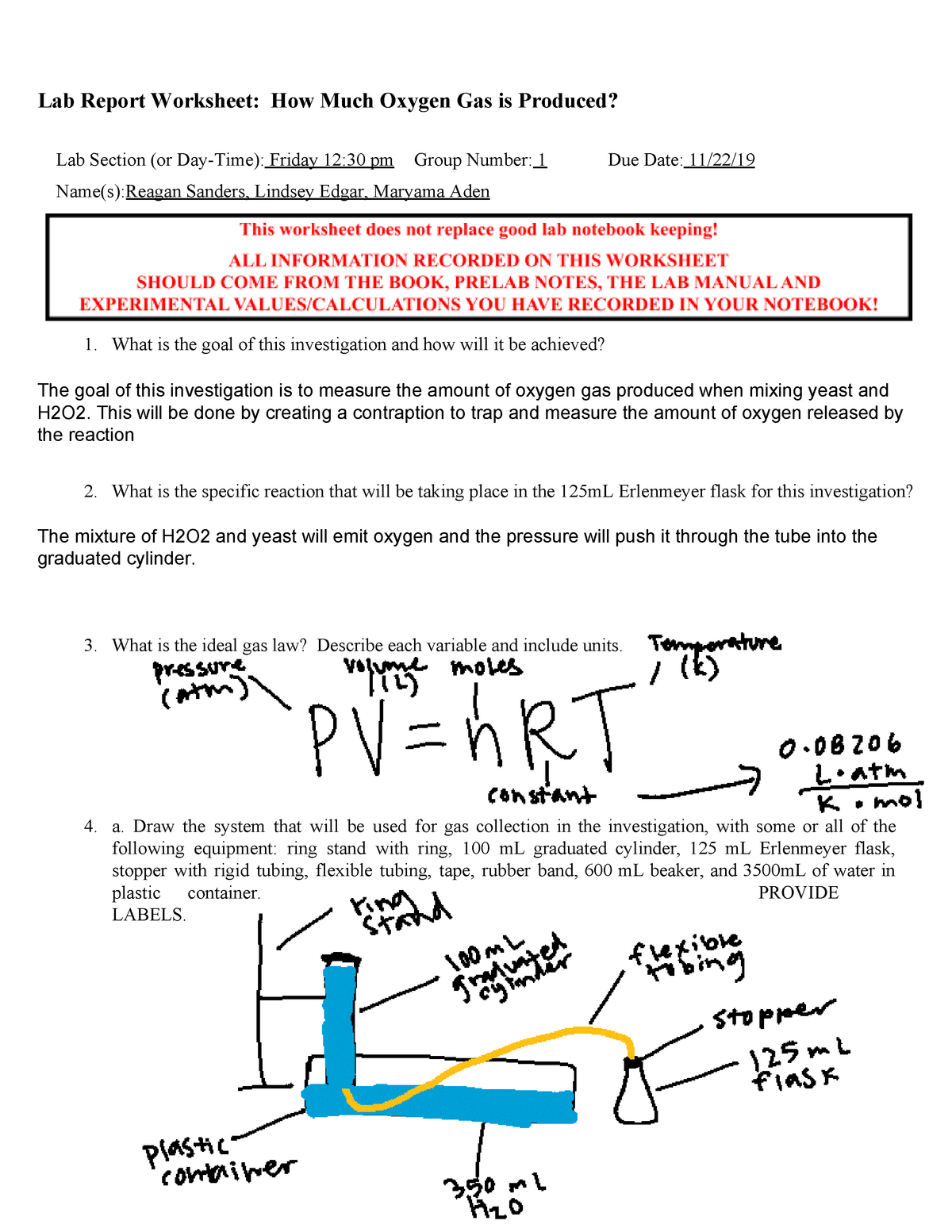
Copy of 101How Much CHM 113 Oxygen Gas is Produced Worksheet RS-1 - Lab Report Worksheet: How Much - Studocu

PDF) Identifying and Engaging the Internal and External Stakeholders, the Outcome and Target Champions and Collaborators

CHM 2106 : 2106 - UG

My publications - CHM 201-LECTURE II-CHEMICAL THERMODYNAMICS
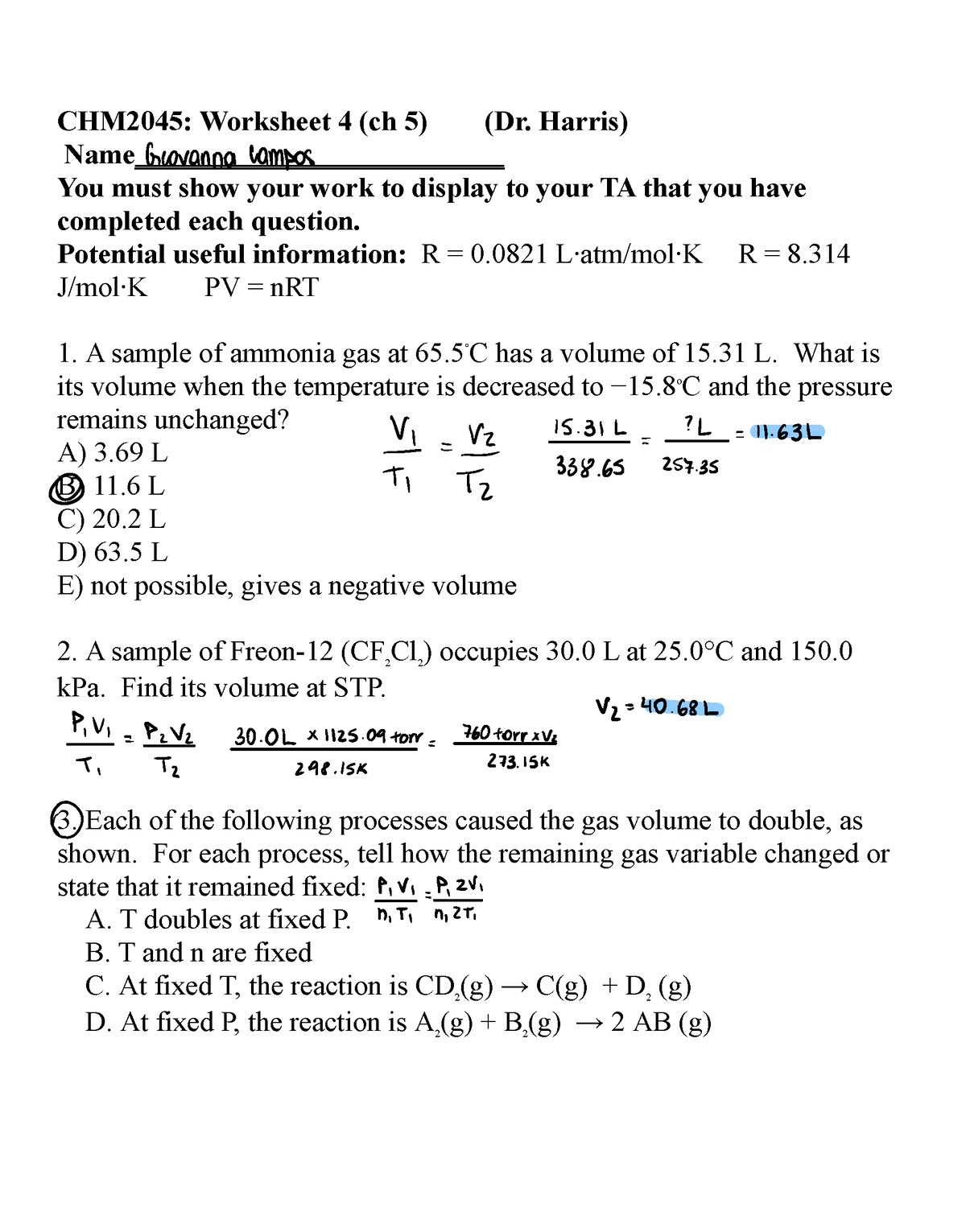
WS 4 Ch - Discussion worksheet for Professor Steven Harris's discussion section - CHM2045: Worksheet - Studocu

Green chemistry-assisted pharmaceuticals—research, revolution, and revenue - Book chapter - IOPscience
Compressibility factor Z - Gaseous State
Real Gases vs Ideal Gases & the Compressibility Factor
How the ideal gas law helped us creating a software tool called
e Compressibility factor (Z) for hydrogen WRT pressure and temperature
Chapter 3 - Physical Properties of Fluids: Gas Compressibility Factor
 Shop The Luxury Collection Hotels Exclusive Bedding, Frette Linens, Bath Essentials and More at The Luxury Collection Store
Shop The Luxury Collection Hotels Exclusive Bedding, Frette Linens, Bath Essentials and More at The Luxury Collection Store Rugby Sevens player Charlotte Caslick and her partner Lewis Holland pose for a photograph at the
Rugby Sevens player Charlotte Caslick and her partner Lewis Holland pose for a photograph at the Yoga Pants Plus Size Casual Solid Color Elastic High Rise Pants for Women Fashion Slim Fit Workout Trendy Womens Pants Flare Lightweight Party
Yoga Pants Plus Size Casual Solid Color Elastic High Rise Pants for Women Fashion Slim Fit Workout Trendy Womens Pants Flare Lightweight Party Bioflect FIR Therapy Lymphedema Micromassage Compression Capri Pants In Orem, Utah
Bioflect FIR Therapy Lymphedema Micromassage Compression Capri Pants In Orem, Utah Strapless Silicone Deep U Bra Self-adhesive Gel Sticky Invisible
Strapless Silicone Deep U Bra Self-adhesive Gel Sticky Invisible Pink Jurassic Jungle Women's Pajama Pants - Little Sleepies
Pink Jurassic Jungle Women's Pajama Pants - Little Sleepies