The Cottrell Experiment and Diffusion Limitation 3/3
4.9 (362) In stock

In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.

Electrochemistry with Stationary Disk and Ring−Disk Millielectrodes in Magnetic Fields

Electrochemistry with Stationary Disk and Ring−Disk Millielectrodes in Magnetic Fields
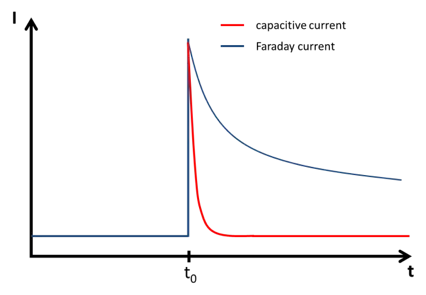
Capacitive Current - PalmSens

Biosensors - PalmSens

An insight into polyscopoletin electrosynthesis by a quality-by-design approach
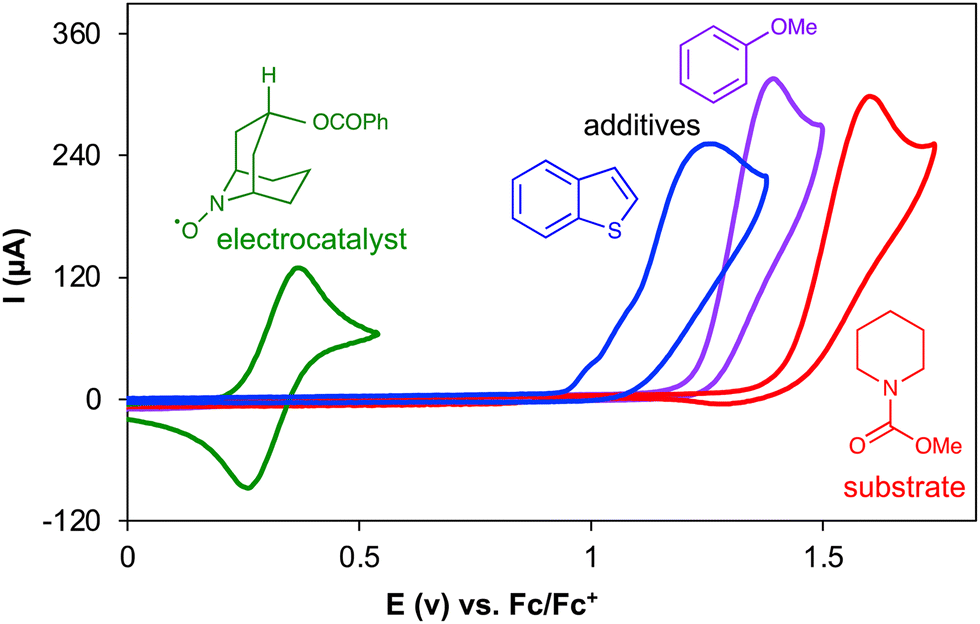
Cyclic voltammetry and chronoamperometry: mechanistic tools for organic electrosynthesis - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D2CS00706A
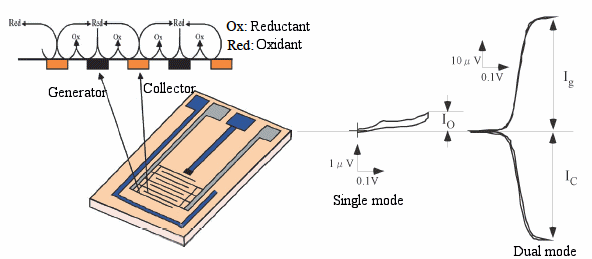
EC_electrode_handbook ALS,the electrochemical company
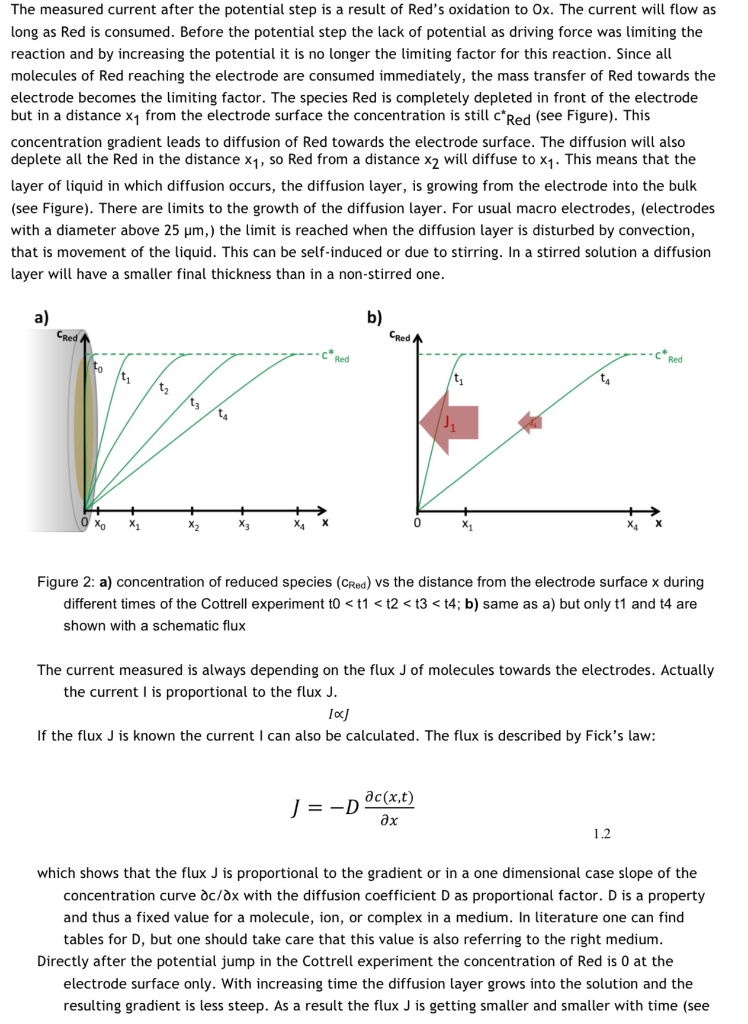
Figure 1.1: Cottrell experiment in KCl solution with

Chapter 3 transport phenomena in electrolytic systems and concentration overpotential. - ppt video online download

The interpretation of small molecule diffusion coefficients: Quantitative use of diffusion-ordered NMR spectroscopy - ScienceDirect

support/electrochemical technique

PDF) Time of Flight Electrochemistry: Diffusion Coefficient Measurements Using Interdigitated Array (IDA) Electrodes
Theory - Chemistry LibreTexts
Spectroscopy of Electrochemical Systems
Double layer forces - Wikipedia
Three models of the electric-double-layer at a positively charged
 Reliability of the AK-47 - the most important reasons - Zbrojownia Modlin
Reliability of the AK-47 - the most important reasons - Zbrojownia Modlin Kay Unger New York Womens Pleated Walk-Thru Formal Jumpsuit Evening BHFO 2117
Kay Unger New York Womens Pleated Walk-Thru Formal Jumpsuit Evening BHFO 2117 Vintage Aussiebum Jockstrap Contour Pouch Lift Pocket S
Vintage Aussiebum Jockstrap Contour Pouch Lift Pocket S Heat Holders Legging thermique extra chaud pour femme (0,63 tog
Heat Holders Legging thermique extra chaud pour femme (0,63 tog- Were the early 2000s (2000 - 2005) more like the late 2000s (2006 - 2009) or the late 90s (1996 - 1999)? - Quora
 Comfort Choice Womens Plus Size Cotton Brief 10
Comfort Choice Womens Plus Size Cotton Brief 10