32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
4.8 (225) In stock
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent
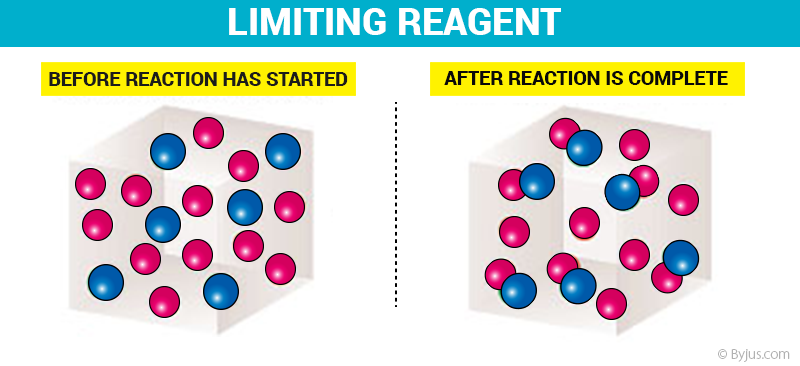
How to find Limiting Reagents? - Detailed Explanation with Examples

Answered: 3. Hydrogen and oxygen gas combine to…

Interface, Vol. 32, No. 2, Summer 2023 by The Electrochemical Society - Issuu
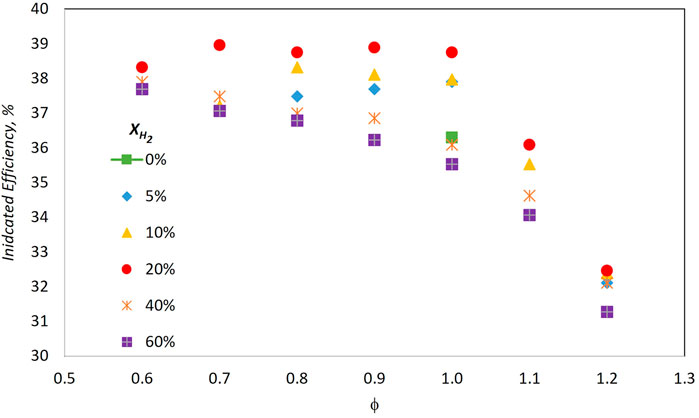
Frontiers Ammonia as Green Fuel in Internal Combustion Engines: State-of-the-Art and Future Perspectives
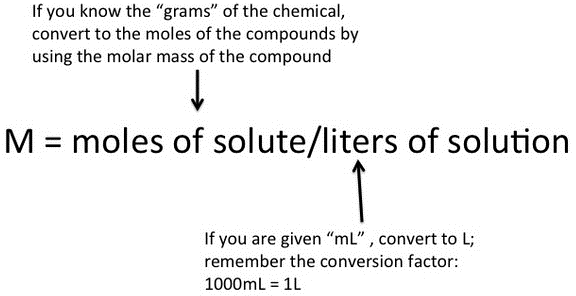
Reaction Stoichiometry – Introductory Chemistry

Q. 88.6 mathrm{g} of mathrm{H}_{2} reacts with 32 mathrm{g} of mathrm{O}_{2} to yield water. Which is the limiting reactant? Find the mass of water produced and the amount of excess reagent left.
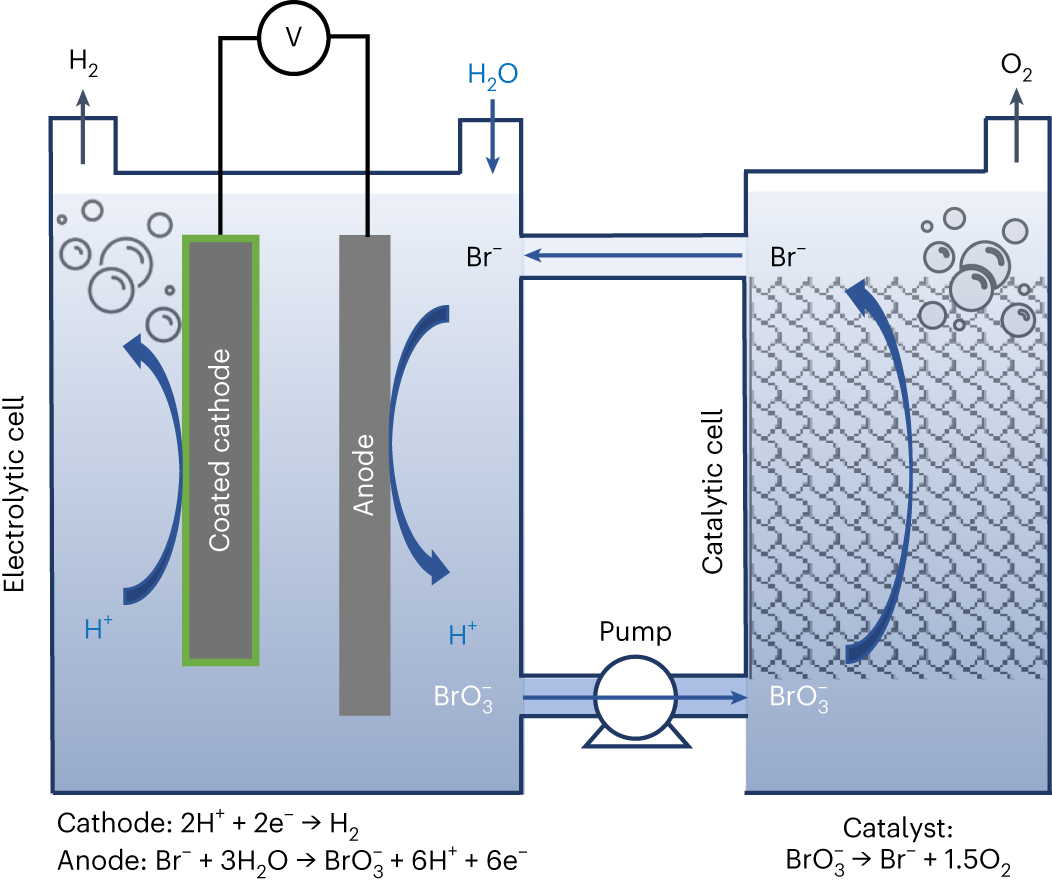
Electrochemical and chemical cycle for high-efficiency decoupled water splitting in a near-neutral electrolyte

Percent Yield Calculator
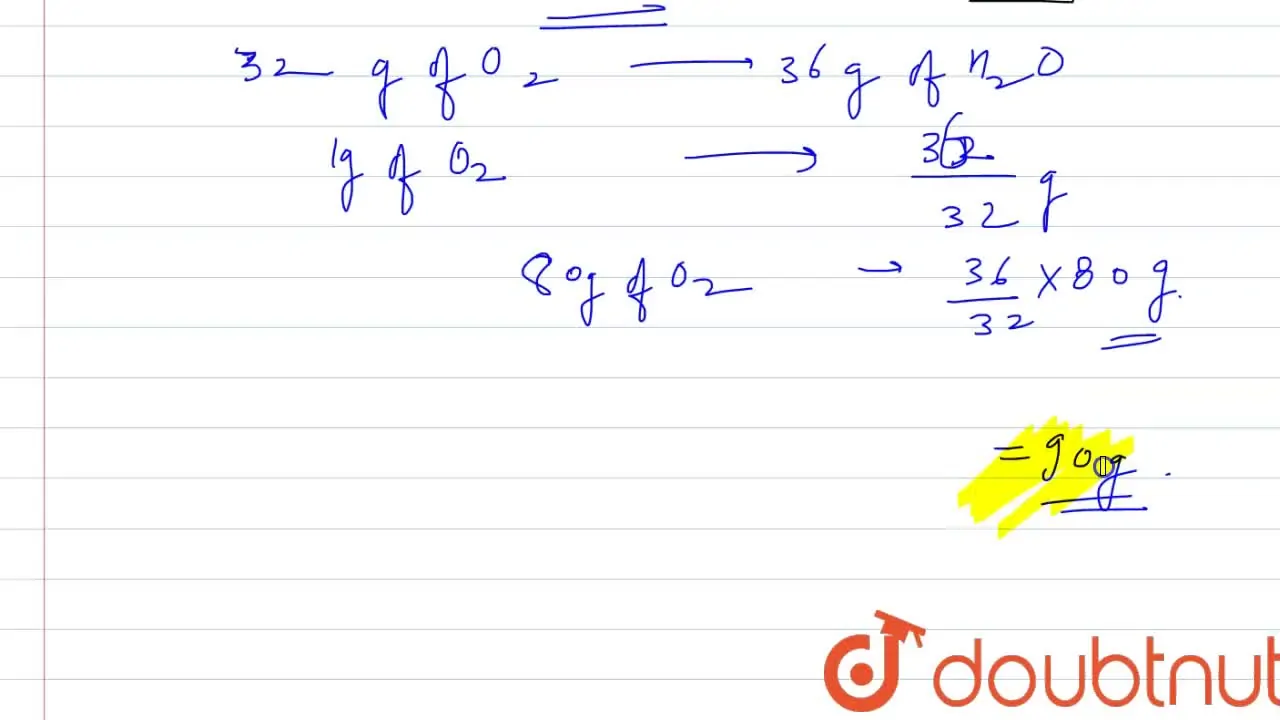
80 g of H(2) is reacted with 80 g of O(2) to form water. Find out the
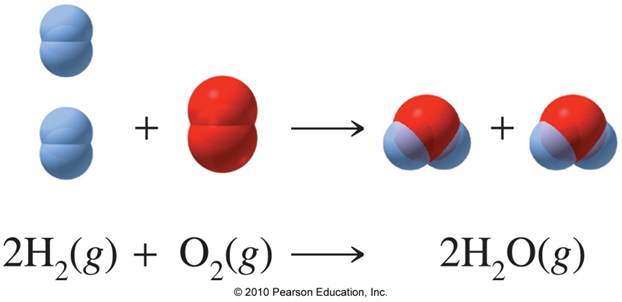
How many grams of water can be produced the combination of 8 grams of oxygen and 8 grams of hydrogen?

Aqueous Transformation of a Metal Diformate to a Metal Dihydride Carbonyl Complex Accompanied by H2 Evolution from the Formato Ligands
Comprar Chocolate Trento Duo 32G Peccin
Hershey's Chocolate Syrup, 32 g : : Grocery & Gourmet Foods
HALLS Rebuçados Morango Sem Açúcar 32 g, CARAMELOS DUROS
 What is Venture Capital? Definition, Benefits, How It Works
What is Venture Capital? Definition, Benefits, How It Works- Warrior Mat curated on LTK
 Wingslove, Intimates & Sleepwear
Wingslove, Intimates & Sleepwear Get Exclusive Marathon Running Apparel from Ultra Marathon Clothing Manufacturer, by Olivia Murphy
Get Exclusive Marathon Running Apparel from Ultra Marathon Clothing Manufacturer, by Olivia Murphy- Secret Fashion Fixes - Online Shop - Voted the best everyday
 KHAITE Nepala diamond-lace Leggings - Farfetch
KHAITE Nepala diamond-lace Leggings - Farfetch
