kinetic theory - Why doesn't Helium behave as an ideal gas
4.8 (573) In stock

I am a bit confused (might be due to some conceptual misunderstanding) as to why doesn't Helium behave as an ideal gas (it shows a deviation from the $pV$ vs $p$ graph)? (Given the fact that it is
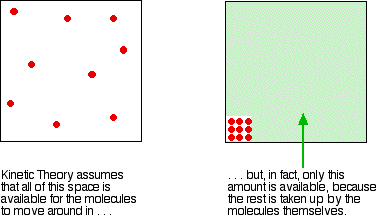
Real gases

Is kinetic theory applicable to ideal gas only? If yes, why is it so? - Quora

SOLUTION: Cbse class 11 physics chapter 13 kinetic theory revision

SOLUTION: Cbse class 11 physics chapter 13 kinetic theory revision notes - Studypool

Fast atom effect on helium gas/graphite interfacial energy transfer - ScienceDirect

The Behavior and Applications of Gases
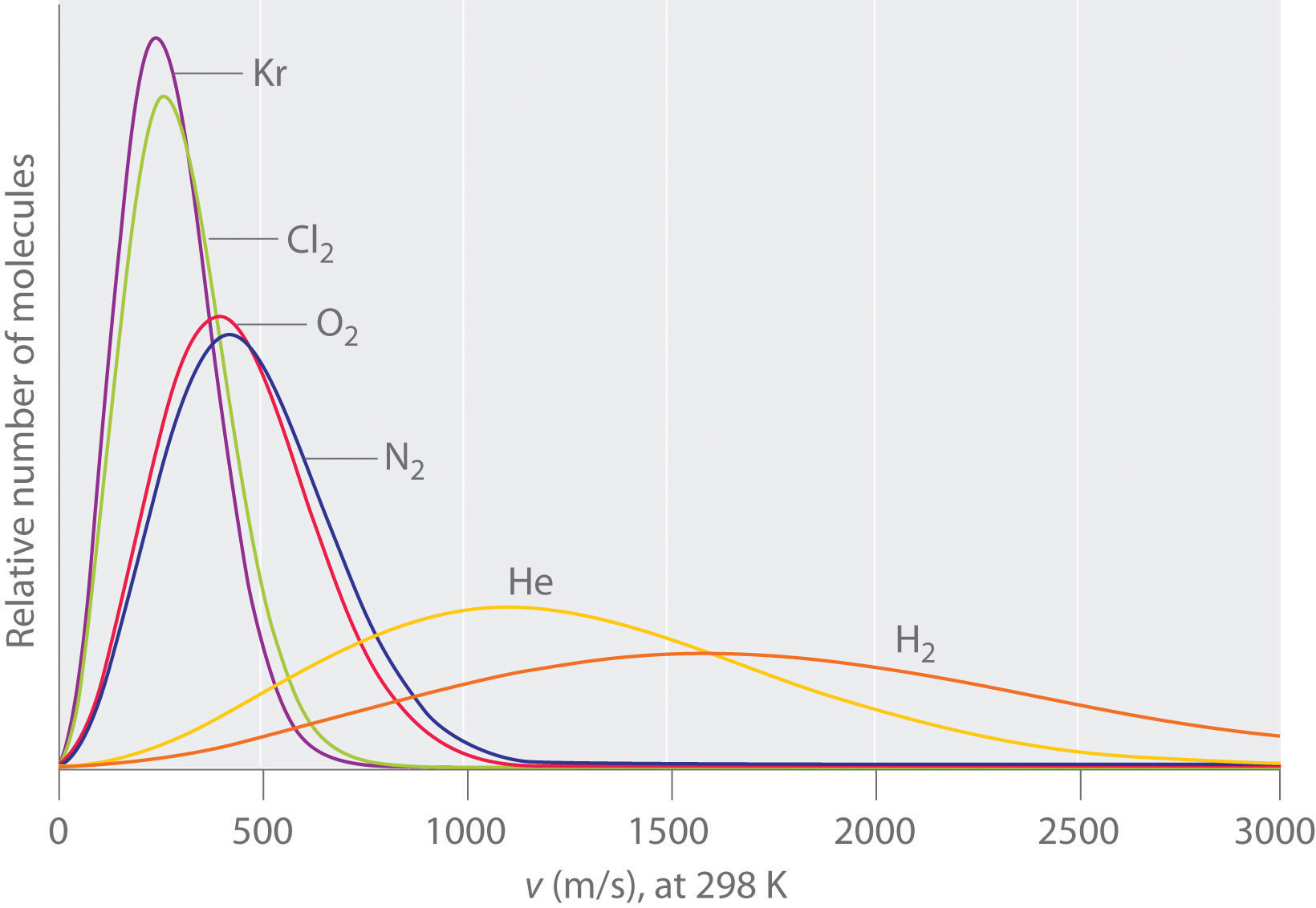
10.7: The Kinetic Theory of Gases - Chemistry LibreTexts
Between N2 and O2, which gas is more ideal? - Quora

Kinetic Theory of Gas - an overview
In kinetic theory, we assume that the number of molecules in a gas

Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature
Ideal gas approximation - Energy Education
Ideal Gas Law: Statement, Characteristics, Formula & Problems
Kinetic Theory of an Ideal Gas: Equation, Assumption, Concept, Examples
 Where 'Gossip Girl' Went, Fashion — and Dollar Signs — Followed - Fashionista
Where 'Gossip Girl' Went, Fashion — and Dollar Signs — Followed - Fashionista Vinyl Pants Pvc Bell-bottom Pants With Medium Waist Facing and
Vinyl Pants Pvc Bell-bottom Pants With Medium Waist Facing and Faithfull the Brand – MERAKIBOUTIQUE
Faithfull the Brand – MERAKIBOUTIQUE SUTIÃ VALENTINA - RUBI LISTRAS
SUTIÃ VALENTINA - RUBI LISTRAS Buy Enamor Women Black Cami Cotton Bra Non Padded Non Wired With
Buy Enamor Women Black Cami Cotton Bra Non Padded Non Wired With Nintendo Switch sales hit 103 million, surpassing Nintendo Wii
Nintendo Switch sales hit 103 million, surpassing Nintendo Wii